infotx@cpt.eurofinsus.com |
(512) 243-6426

Surgical Hand Scrub or Rub Testing at ECRL
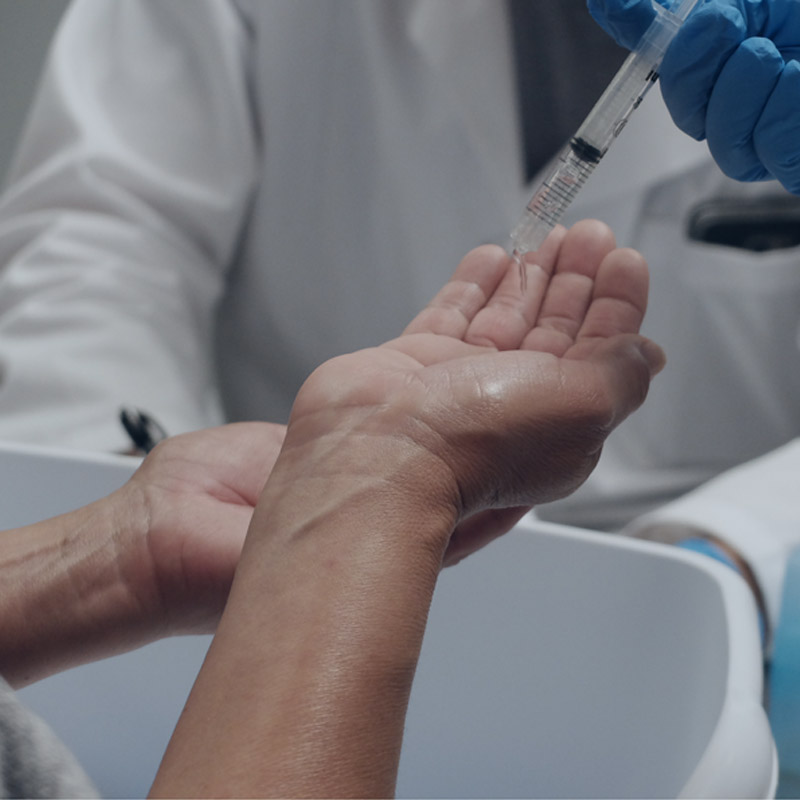
Surgical hand scrubs or surgical hand rubs are fast acting broad spectrum health care antiseptic rub (i.e. leave-on) or wash (i.e. rinse-off) products designed to significantly reduce the number of microorganisms on the skin prior to surgery.
Surigical hand scrub or rub testing measures the reduction of immediate and persistent bacteria on hands after single or multiple treatments using surgical hand hygiene products. The testing of a surgical hand scrub or surgical hand rub proposed in the 1994 Tentative Final Monograh involved multiple test product uses and the repeated measurement of bacterial reductions to determine both intermediate and persistent antimicroial activity. Additionally the effectiveness of the product to suppress bacterial growth over the course of 6 hours is documented. While the new proposed guidance for health care antiseptics retains the effectiveness critera, the FDA is no longer requiring that these products demonstrate a cumulative antiseptic effect.
Surgical Scrub or Rub Testing at A Glance
For this testing a supervised surgical scrub or rub is completed before one hand for each subject is immediately tested. 6 hours after the surgical scrub or treatment the second hand is tested for surviving microorganisms. If the cumulative antibacterial effect of the surgical scrub or rub is to be evaluated, testing may be continued over the course of a 5-day period to profile the cumulative effect of using the product.
FDA Clinical Testing Bacterial Log10 Reduction Effectiveness Criteria in the Recent Proposed Rule vs. the 1994 Tentative Final Monograph
The FDA has recently revised the product performance criteria for surgical hand scrubs. FDA has determined that it is the critical element of effectiveness is that the product is
effective after the first application, since this is representative of real world use conditions. Therefore the following product performance criteria outlined in the recent tentative guidance are only for a single-product application.
Active Ingredients Eligible for OTC Drug Review
Each of the following active ingredients has demonstrated to be eligible for OTC Drug Review for use as a health care personnel surgical hand scrub.