infotx@cpt.eurofinsus.com |
(512) 243-6426
ASTM E1173 Evaluation of Preoperative, Precatheterization, or Preinjection Skin Preparations
The ASTM E1173 test measures the immediate and/or persistent reduction of microflora on the skin after using Preoperative, Precatheterization, or Preinjection skin products. A cup scrub technique is used to collect microbial samples from relevant skin areas (inguinal region, abdomen, subclavian region, or median cubital region) depending on the product to be tested. Microbial counts are obtained before and after antimicrobial use on the same skin site. A significant reduction in the number of microorganisms from the baseline level demonstrates product antimicrobial efficacy.
ASTM E1173: Procedure At a Glance
In these studies, healthy subject individuals with normally high levels of microflora on the skin sites to be tested (3.0 log10/cm2 for preoperative and precatheterization testing, 1.0 log10/cm2 for preinjection testing) are chosen from a group of study participants who have not used any topical or oral antimicrobials for at least two weeks preceding the test. Baseline pretesting is completed at least 72 hours before product testing, and baseline samples are collected immediately before applying the antimicrobial test product.
The testing procedures follow protocols which vary depending on the product type. For preoperative product testing, a minimum of three microbial samples are collected from specified skin sites, typically within six hours of treatment with the antimicrobial test product. When precatheterization products are tested, three samples are collected between 30 seconds and 24 hours post treatment. For preinjection antiseptic testing, a microbial sample is collected immediately (30 seconds) following treatment with the antimicrobial product. These test often require sampling from what the method refers to as moist and dry skin sites, where sites such as the inguinal region and the median cubital region serve as moist skin sites and sites such as the abdomen and subclavian region serve as dry skin sites.
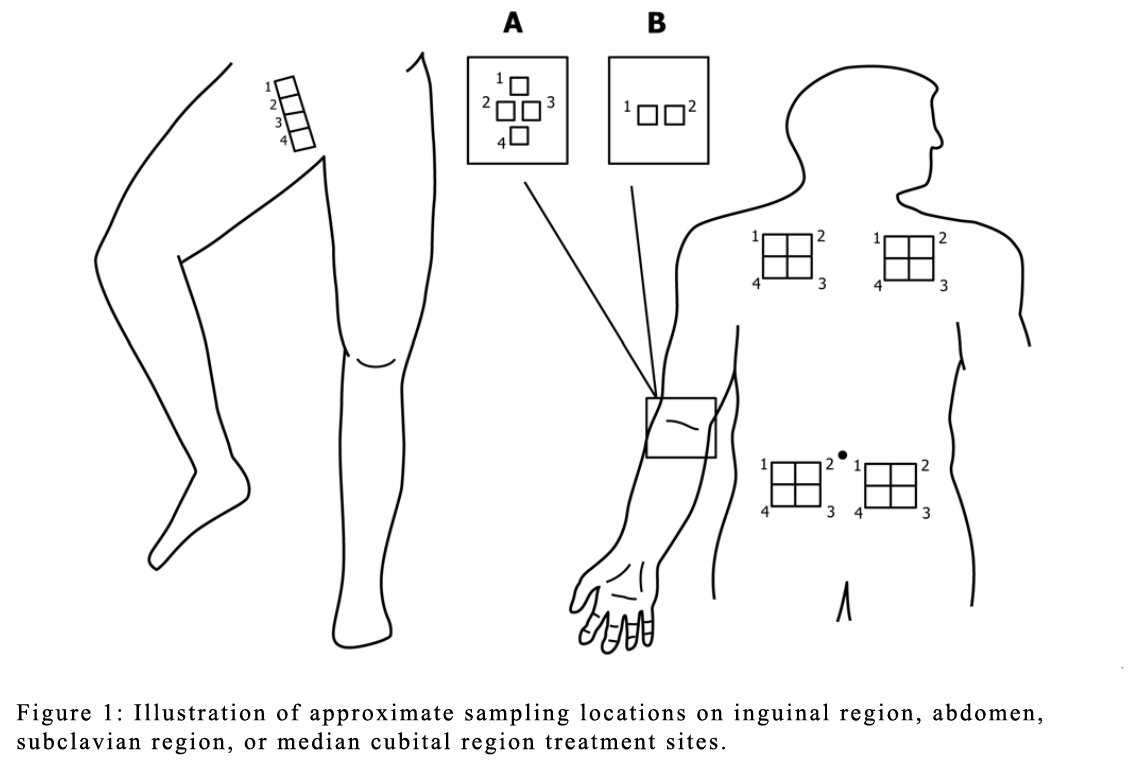
The bacteria obtained using cup scrub techniques are enumerated using standard procedures, and the number of surviving microorganisms are determined. This study design allows for statistical evaluation using either parametric or non-parametric models.
ASTM E1173: EUROFINS CRL’S EXPERTISE
Eurofins CRL was founded in 2017 as a collaborative effort between two specialty contract testing facilities. ECRL combines the expertise derived from its legacy with the innovative methods and quality focus that makes the Eurofins name a leader in third-party testing world-wide.
Eurofins CRL Is:
A contract testing laboratory designed to support the hand hygiene and infection control industry. Testing is carried out in compliance with current FDA, Good Laboratory Practice (GLP), and Good Clinical Practice (GCP) regulations by skilled, experienced microbiologists and chemists, to quickly meet your project testing goals.