infotx@cpt.eurofinsus.com |
(512) 243-6426
ASTM E2276 Determining the Bacteria-Eliminating Effectiveness of Hygienic Handwash and Handrub Agents Using the Fingerpads of Adults
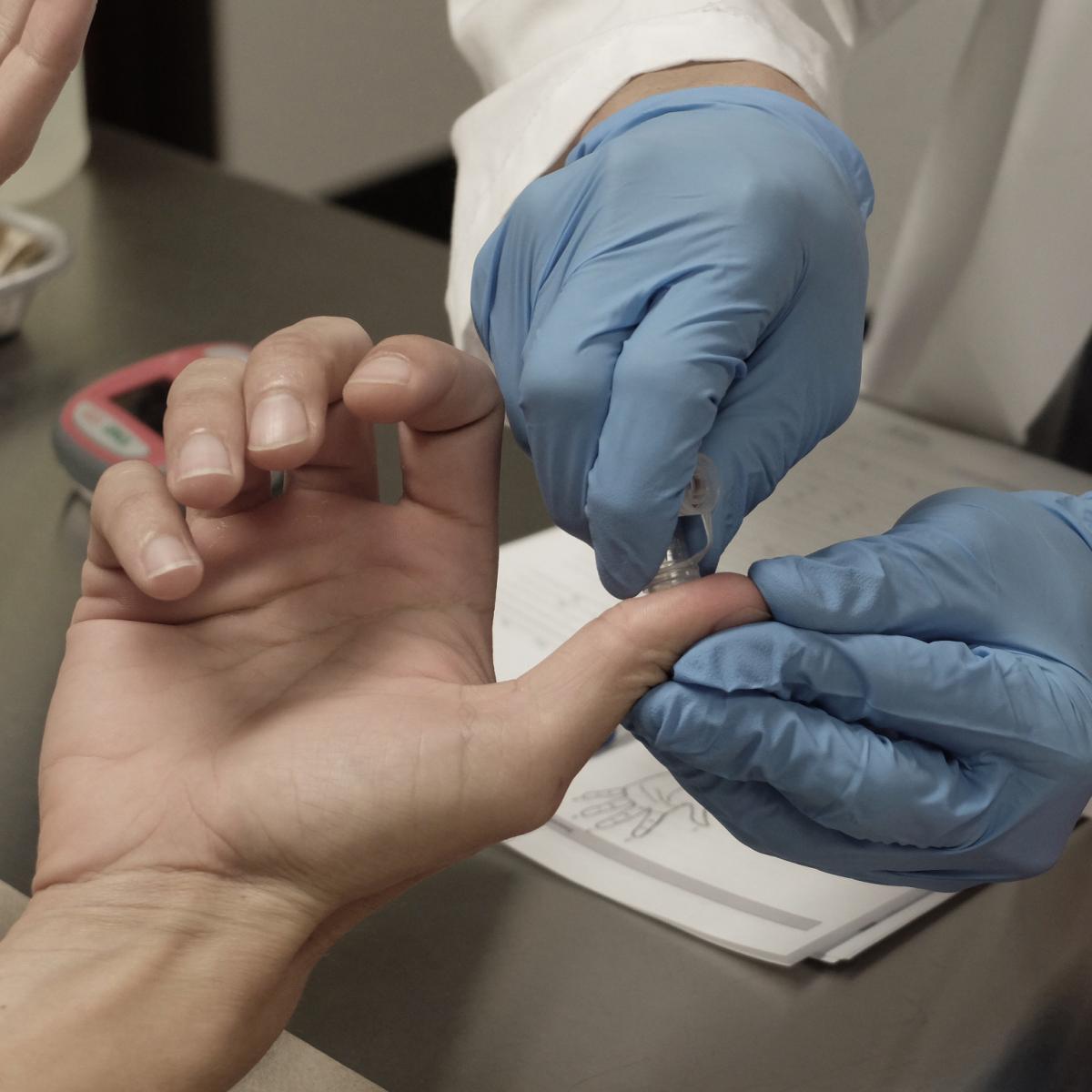
The ASTM E2276 test evaluates the effectiveness of hand wash and hand rub formulations for inactivating and/or mechanically removing transient bacteria from the hands of health care workers. Typically, fingerpads which have been contaminated with a bacterial inoculum suspension are exposed to a hand wash or hand rub product, and the efficacy of the product is determined.
ASTM E2276: Procedure At a Glance
This test is conducted on at least three healthy study participants with undamaged skin who have not used any antimicrobial products for at least one week before testing.
Each subject washes their hands with a non-antimicrobial soap for at least ten seconds and then rinses and dries their hands. The hands are further pre-cleaned using a 70% ethanol cleansing process. After defining an area on each finger pad, a test bacterial suspension, with or without a soil load, is used to contaminate the pads with at least 105 infectious species. Typical bacteria used include Serratia marcescens, Escherichia coli, Acinetobacter baumannii, Staphylococcus aureus, and Staphylococcus epidermidis. The initial contamination level is measured by determining the amount of bacteria deposited on each thumb pad prior to drying.
For the actual test measurements, the suspension is allowed to dry on each of the finger pads. The contaminated finger pad areas are then exposed either to a control or to a hand wash/hand rub product following a defined process. For hand wash testing, there is a simulated water rinse step. This step is not used for testing hand rub products.
The microorganisms surviving on the finger pads are collected via extraction and the eluates are assayed following standard procedures. Input, drying, and viability controls are included in this procedure. The baseline and test substance bacteria levels are compared and the reduction of the bacterial level that can be attributed to the use of the hand wash or hand rub product is reported. Note that this test method is not designed to be used to evaluate the effectiveness of surgical hand scrubs or preoperative skin preparations.
ASTM E2276: EUROFINS CRL’S EXPERTISE
Eurofins CRL was founded in 2017 as a collaborative effort between two specialty contract testing facilities. ECRL combines the expertise derived from its legacy with the innovative methods and quality focus that makes the Eurofins name a leader in third-party testing world-wide.
Eurofins CRL Is:
A contract testing laboratory designed to support the hand hygiene and infection control industry. Testing is carried out in compliance with current FDA, Good Laboratory Practice (GLP), and Good Clinical Practice (GCP) regulations by skilled, experienced microbiologists and chemists, to quickly meet your project testing goals.